Abstract
Background
An association has been identified between the diagnosis-to-treatment interval (DTI) and prognostic clinical factors and outcomes in patients with previously untreated diffuse large B-cell lymphoma (DLBCL) (Maurer et al, JCO 2018). This association can result in inadvertent selection bias in clinical trials to exclude patients with aggressive disease due to the inability to delay treatment long enough to fulfill enrollment criteria, compromising the validity of clinical trial study results to the general population. A similar concern exists in the relapsed/refractory (r/r) DLBCL setting regarding patient selection bias against patients with aggressive disease requiring immediate treatment following relapse or progression. Here we examine the time from progression after immunochemotherapy (IC) to the initiation of first salvage chemotherapy and its association with outcome.
Patients and Methods:
Newly diagnosed patients were prospectively enrolled within 9 months of diagnosis in the University of Iowa/Mayo Clinic Lymphoma SPORE Molecular Epidemiology Resource (MER), now a subcohort of the Lymphoma Epidemiology of Outcomes (LEO) Cohort Study, and followed for progression/relapse, retreatment, and death. This analysis includes patients with their first r/r DLBCL following frontline IC who initiated aggressive salvage chemotherapy as identified and included in a previous publication (Farooq et al, BJH 2017). The progression-to-treatment interval (PTI) was defined as the time in days from date of progression from IC to initiation of salvage therapy. The date of progression was defined as either a) the date biopsy was obtained for patients who had a biopsy to confirm progressive disease or b) the date of the scan or clinical examination indicating progression in patients who did not have a biopsy performed. Event-free survival (EFS) was defined as time from initiation of first salvage therapy to progression or relapse, initiation of new anti-lymphoma therapy, or death due to any cause; overall survival (OS) was defined as time from initiation of salvage therapy until death due to any cause.
Results:
162 patients with r/r DLBCL enrolled in the MER from 2002-2012 who initiated aggressive salvage chemotherapy with intent to transplant and had confirmed dates for both progression after IC and start of salvage therapy were evaluated. Median age at first progression for these patients was 64 years (range 36-76) and 104 (64%) were male. Median time from diagnosis to first progression on IC was 6.7 months (IQR: 4.6-12.7). Initial salvage therapy was R-ICE (80%), R-DHAP (8%), rituximab, oxaliplatin, cytosine arabinoside, and dexamethasone (ROAD) (6%), and other (7%). At a median follow-up of 49 months from initiation of salvage therapy (IQR: 33-74), 116 patients had died (72%).
Median PTI was 6 days (IQR: 2-13). 110 patients (68%) had biopsy confirmation of disease prior to initiating salvage therapy; median PTI was 2 days (IQR: 1-7) for patients who had biopsy confirmation vs. 7 days (IQR: 3-16) in patients without biopsy confirmation, Wilcoxon p=<0.0001. There was no difference in OS from initial salvage therapy between patients who did not have biopsy confirmation (HR=1.09, 95% CI: 0.74-1.60, p=0.68) compared to patients with biopsy confirmation.
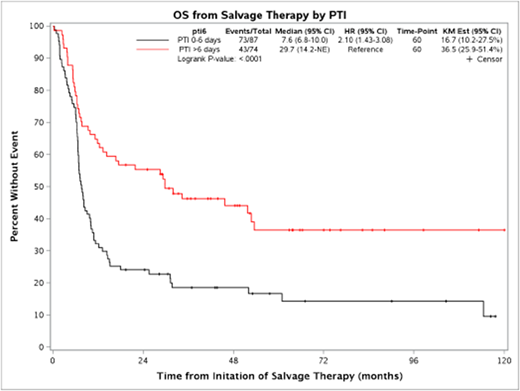
Patients with short PTI (0-6 days) had significantly worse overall survival from initiation of salvage therapy (median OS = 7.6 months, HR=2.10 (95% CI: 1.43-3.08) compared to patients who initiated therapy 7 or more days from progression (median OS = 29.7 months), logrank p<0.0001. This association remained consistent (HR=2.20, 95% CI: 1.48-3.26, p<0.0001) after adjusting for age and biopsy confirmation of progression. Short PTI was also associated with inferior response rate to initial salvage therapy (51% vs. 65%, p=0.058), lower rate of proceeding to transplant after initial salvage therapy (33% vs. 55%, p=0.0063), lower rate of being event-free 24 months from initial salvage therapy (16% vs 33%, p=0.011) and lower rate of ever proceeding to transplant (44% vs. 59%, p=0.057).
Conclusions:
A short progression-to-treatment interval is strongly associated with inability to proceed to transplant and inferior overall survival in r/r DLBCL. These results have implications for the design and interpretation of clinical trials in the relapsed/refractory setting.
Maurer:Celgene: Research Funding; Nanostring: Research Funding; Morphosys: Research Funding. Ansell:Trillium: Research Funding; Merck & Co: Research Funding; Affimed: Research Funding; Bristol-Myers Squibb: Research Funding; Seattle Genetics: Research Funding; Regeneron: Research Funding; LAM Therapeutics: Research Funding; Pfizer: Research Funding; Celldex: Research Funding; Takeda: Research Funding. Witzig:Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Cerhan:Nanostring: Research Funding; Celgene: Research Funding; Jannsen: Other: Scientific Advisory Board.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal